Humira And Crohn's Disease
Humira and crohn's disease. I wish Crohns was a fatal diseaseIm tired Im drained. As a young woman Porche began experiencing symptoms of Crohns disease. HUMIRAs ability to effectively treat the chronic symptoms of Crohns disease makes it an important option for gastroenterologists and patients looking for improved disease management.
User Reviews for Humira to treat Crohns Disease Humira has an average rating of 50 out of 10 from a total of 49 ratings for the treatment of Crohns Disease. Humira adalimumab is another monoclonal antibody and TNF inhibitor that is used to treat people with IBD. Adalimumab was associated with steroids in 28 of cases and with immunomodulators in 10 of patients.
A cohort of 60 CD patients treated with adalimumab between 2005 and 2013 were reviewed for the study. Read more about Porches story on living with Crohns disease. Adalimumab treatment led to growth velocity normalization.
It is approved for adults and children over the age of 6 that have ulcerative colitis or Crohns disease. Efficacy and safety profiles of prolonged adalimumab treatment in children with Crohns disease were consistent with IMAgINE 1 and adult Crohns disease adalimumab trials. The median age was 33 years and the median disease duration was 86 years.
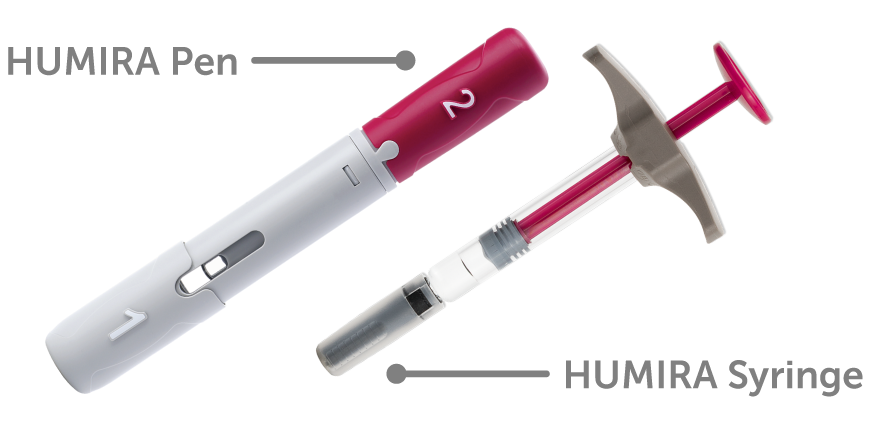
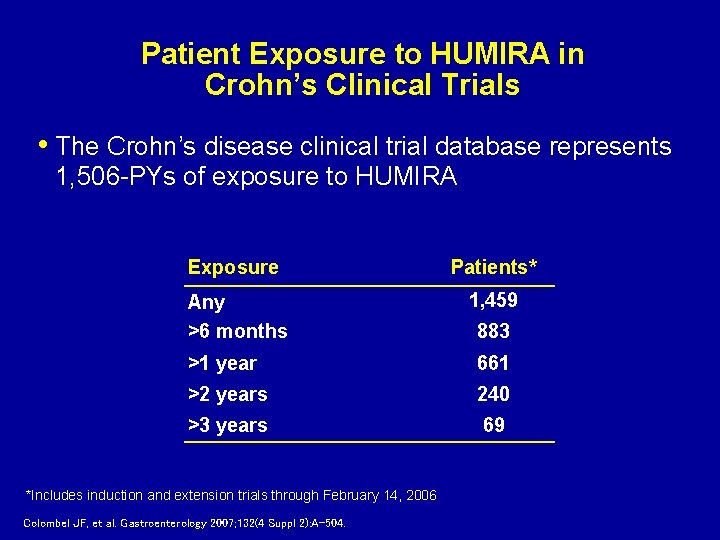
Our aim was to identify an optimal adalimumab level to achieve endoscopic healing in Crohns disease CD. Humira has an average rating of 75 out of 10 from a total of 50 ratings for the treatment of Crohns Disease Maintenance. Humira was initially approved in 2002 and expanded for use in Crohns disease in 2007 and ulcerative colitis in 2012.
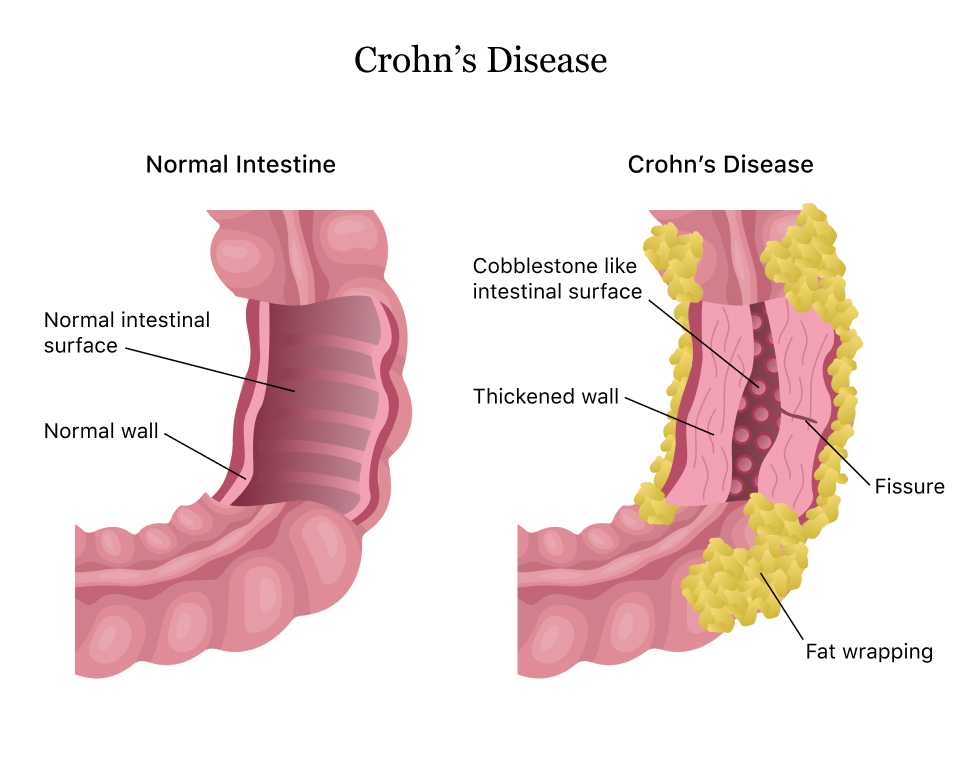
39 of those users who reviewed Humira reported a positive effect while 45 reported a negative effect. JNJs Stelara Fails to Beat AbbVies Humira In Crohns Disease Patients The Janssen Pharmaceutical Companies a unit of Johnson Johnson NYSE. In autoimmune diseases like rheumatoid arthritis psoriatic arthritis Crohns disease psoriasis and the other conditions Remicade and Humira are approved to treat the immune system mistakes the bodys own cells for foreign ones and attacks them causing inflammation pain tissue destruction and other associated symptoms.
While it is reassuring that at-risk people on certain Crohns and Colitis medicines appear to be coping well with coronavirus there is no evidence that taking adalimumab infliximab corticosteroids tacrolimus or tofacitinib for Crohns or Colitis will protect you from coronavirus. Within the 14 weeks after adalimumab optimization 14 patients 333 achieved clinical remission Crohns disease activity index.
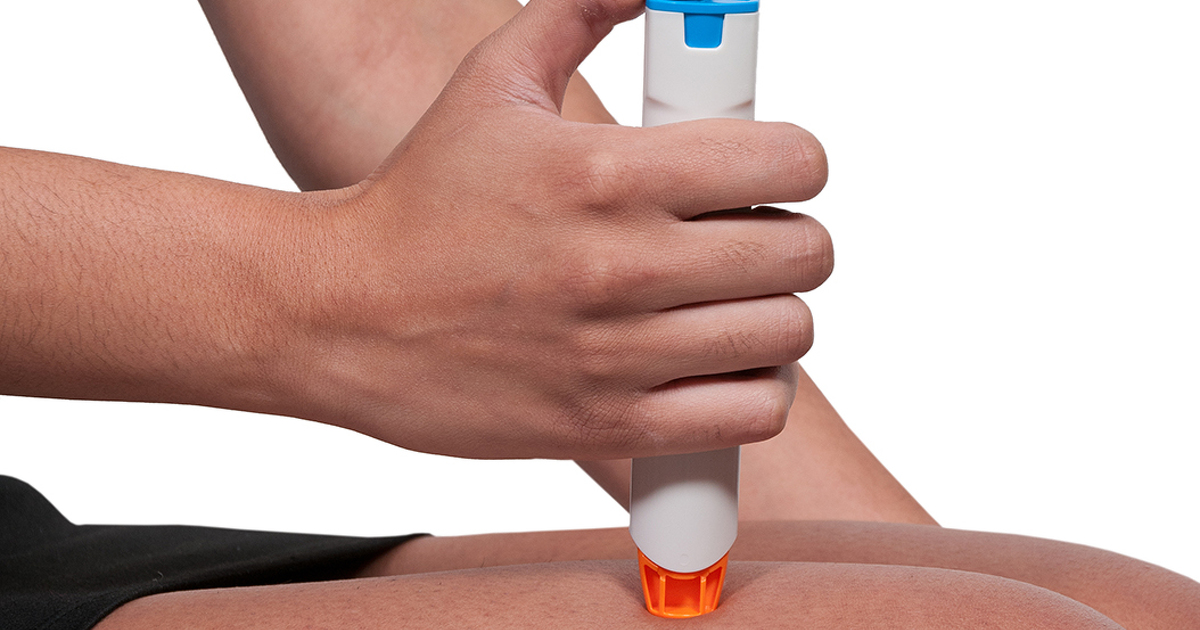
Humira is used to treat many inflammatory conditions in adults such as rheumatoid arthritis psoriatic arthritis ankylosing spondylitis plaque psoriasis and a skin condition called hidradenitis suppurativa.
72 of those users who reviewed Humira reported a positive effect while 16 reported a negative effect. JNJs Stelara Fails to Beat AbbVies Humira In Crohns Disease Patients The Janssen Pharmaceutical Companies a unit of Johnson Johnson NYSE. No new safety signals were identified. Within the 14 weeks after adalimumab optimization 14 patients 333 achieved clinical remission Crohns disease activity index. Efficacy and safety profiles of prolonged adalimumab treatment in children with Crohns disease were consistent with IMAgINE 1 and adult Crohns disease adalimumab trials. Redness pain swelling or itching at the injection site upper respiratory infections such as the common cold headache rash sinus infection nausea abdominal pain back pain urinary tract infection high blood pressure serious allergic reaction rash. User Reviews for Humira to treat Crohns Disease Humira has an average rating of 50 out of 10 from a total of 49 ratings for the treatment of Crohns Disease. Humira has an average rating of 75 out of 10 from a total of 50 ratings for the treatment of Crohns Disease Maintenance. A cohort of 60 CD patients treated with adalimumab between 2005 and 2013 were reviewed for the study.
Prior studies have suggested that adalimumab levels of 49 µgmL are required to achieve clinical remission. The median age was 33 years and the median disease duration was 86 years. A cohort of 60 CD patients treated with adalimumab between 2005 and 2013 were reviewed for the study. 72 of those users who reviewed Humira reported a positive effect while 16 reported a negative effect. JNJs Stelara Fails to Beat AbbVies Humira In Crohns Disease Patients The Janssen Pharmaceutical Companies a unit of Johnson Johnson NYSE. Humira was initially approved in 2002 and expanded for use in Crohns disease in 2007 and ulcerative colitis in 2012. I know these thoughts are bad to have I used to fight them off easily but I dont have the energy anymore to push them away everyday and pretend like life is good and everything is normal when it has been going downhill for years and no signs of improvement in sight.





























Post a Comment for "Humira And Crohn's Disease"