Parkinson Disease Stem Cell Clinical Trials
Parkinson disease stem cell clinical trials. An overview of the latest news in Parkinson disease reported. The trial includes a screening period of up to 4 weeks a 32-week treatment period. Cyto Therapeutics is the wholly owned subsidiary of International Stem Cell Corporation conducting the clinical trial for Parkinsons disease in Australia.
Clinical Study of the Safety and Efficacy of Autologous Neural Stem Cells in the Treatment of Parkinsons Disease. 2010 and Ethical Issues in Clinical Research 2010. Friday January 15 2021.
Resolving ethical issues in stem cell clinical trials. A gene therapy consisting of pluripotent stem cell. This follows the successful restoration of brain cell function in monkeys using these stem cells reported last year.
News in Context. A landmark clinical trial was announced recently by researchers at Kyoto University. Clinical trials using ESCs are already ongoing in Australia NCT02452723 and China NCT03119636 but these are transplants of neural stem cells not DA neurons and are expected to have cytokine effects ie neuroprotection and suppression of inflammation.
Resolving Ethical Issues in Stem Cell Clinical Trials. Bernard Lo MD is a Professor of Medicine and Director of the Program in Medical Ethics at UCSF. The Example of Parkinson Disease.
For the first time in history this trial tests the use of stem cells for the treatment of a major degenerative brain disease. Stem cell-based therapies for Parkinsons disease are moving into a new and exciting era with several groups pursuing clinical trials with pluripotent stem cell PSC-derived dopamine neurons. Gene Therapy Clinical Trials FDA.
Worlds first clinical trial to treat Parkinsons disease with stem cells Researchers from Kyoto University in Japan started a clinical trial this month to treat Parkinsons disease with reprogrammed stem cells. The first patient has been dosed in a still-recruiting Phase 1 clinical trial testing DA01 an investigational cell therapy for Parkinsons disease.
At the same time this is a complex and still insufficiently explored process.
For many myself included the term stem cells meant only embryonic stem cells and evoked controversy. A friend of mine sent me an article about a self-funded clinical trial using stem cells to treat a patient named George Lopez who has Parkinsons. Cyto Therapeutics is the wholly owned subsidiary of International Stem Cell Corporation conducting the clinical trial for Parkinsons disease in Australia. The TRANSNEURO Consortium shares valuable insights that may facilitate planning of human pluripotent stem-cell-derived dopamine cell transplants for future clinical trials on Parkinsons disease. The significance of a trial like this should not be understated as the therapeutic potential of stem cells in. Bernard Lo MD is a Professor of Medicine and Director of the Program in Medical Ethics at UCSF. A recent approval from the US. Latest News in Parkinson Disease. Friday January 15 2021.
The trial includes a screening period of up to 4 weeks a 32-week treatment period. One-Patient Parkinsons Stem Cell Trial. Estimated Primary Completion Date. For many myself included the term stem cells meant only embryonic stem cells and evoked controversy. Clinical trials using ESCs are already ongoing in Australia NCT02452723 and China NCT03119636 but these are transplants of neural stem cells not DA neurons and are expected to have cytokine effects ie neuroprotection and suppression of inflammation. Friday January 15 2021. Latest News in Parkinson Disease.
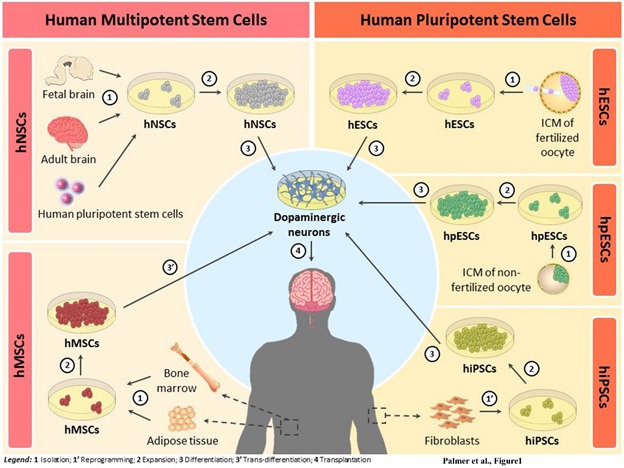



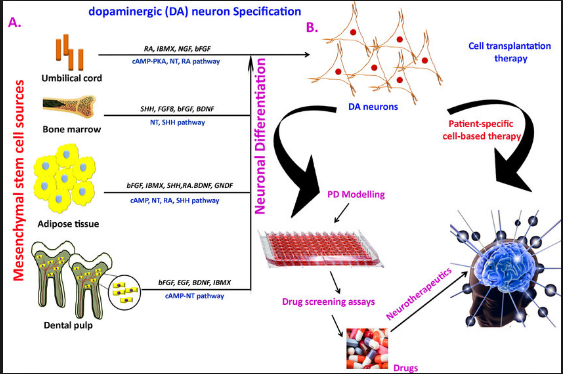





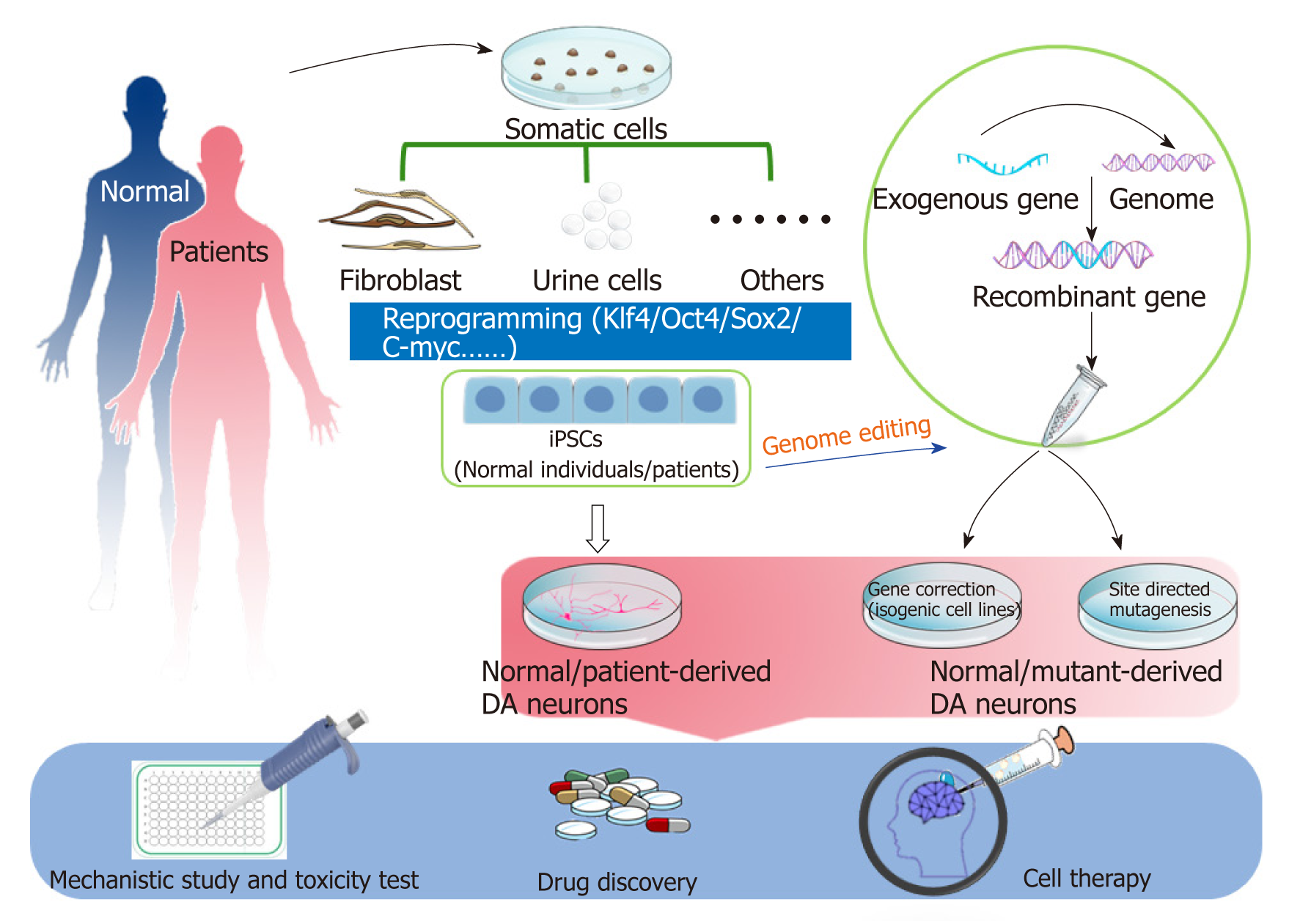
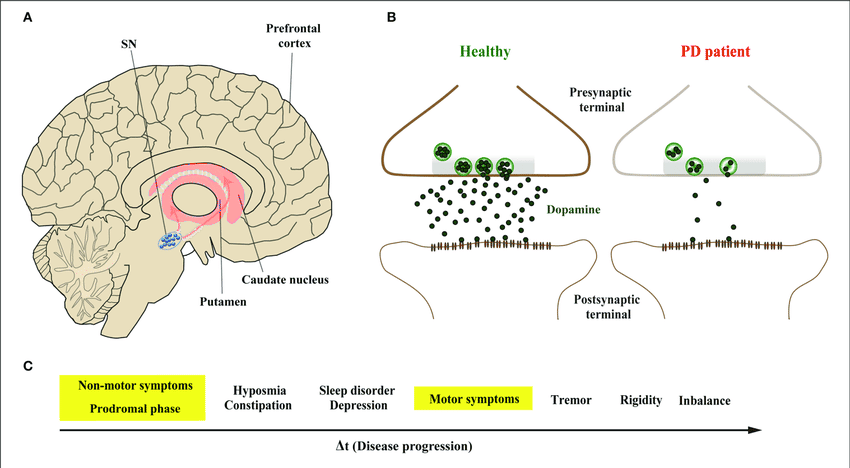

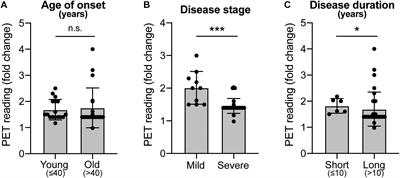
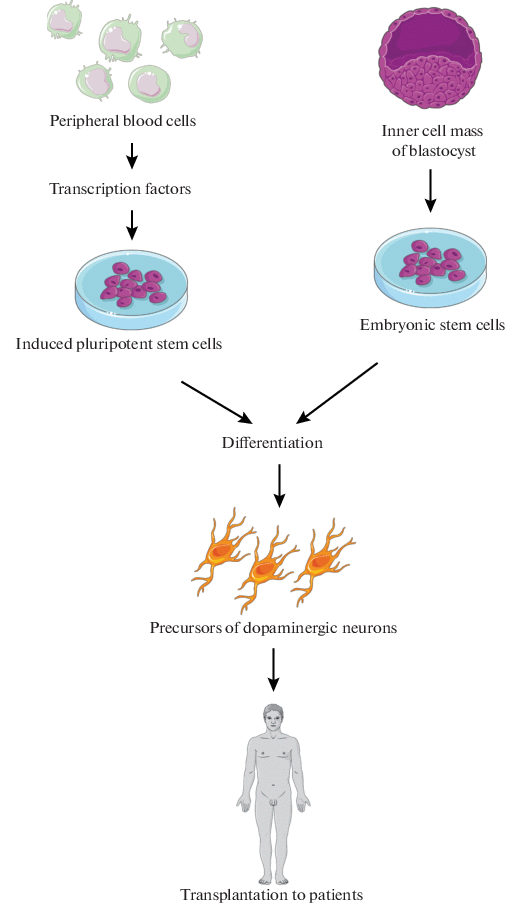

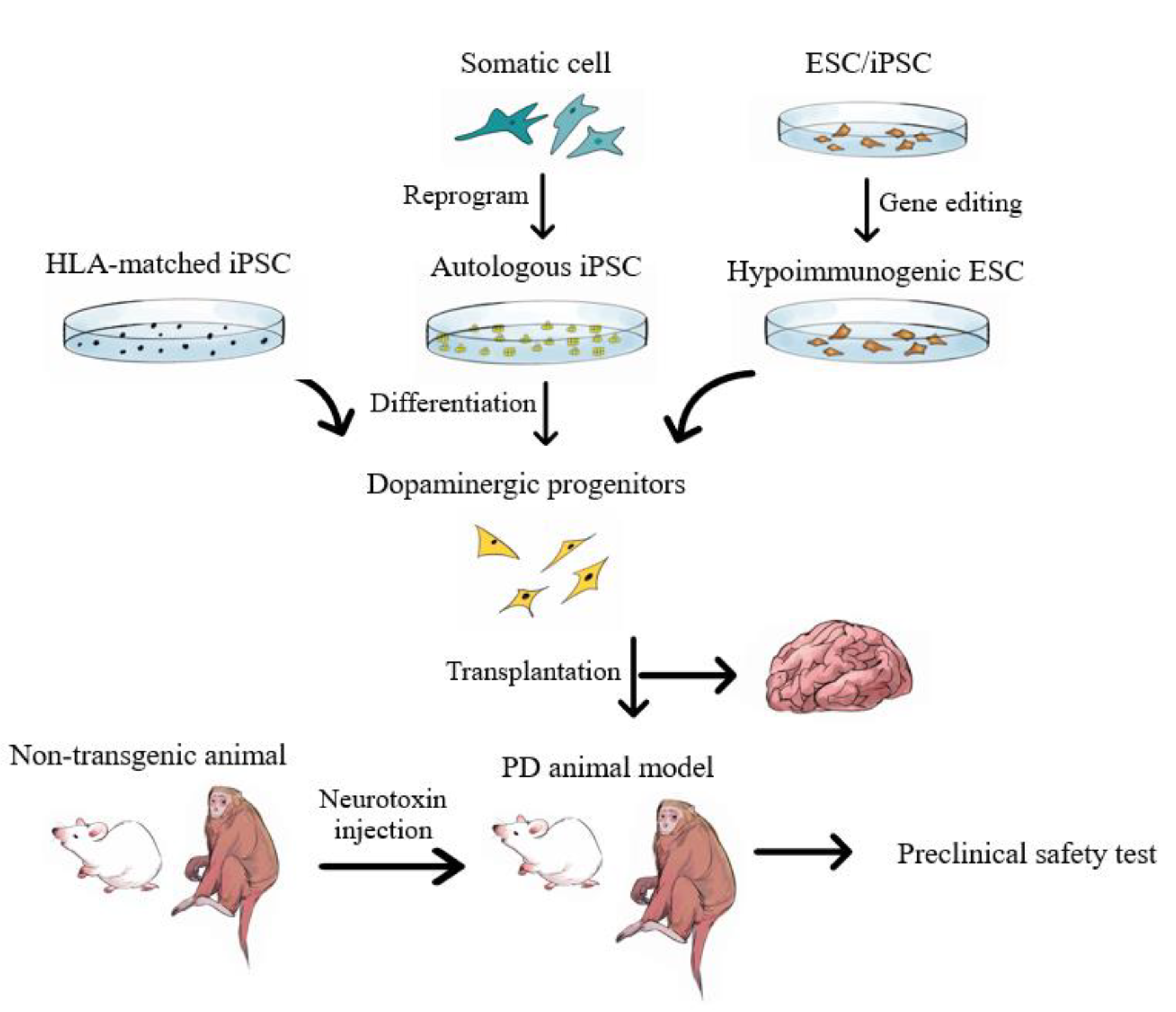
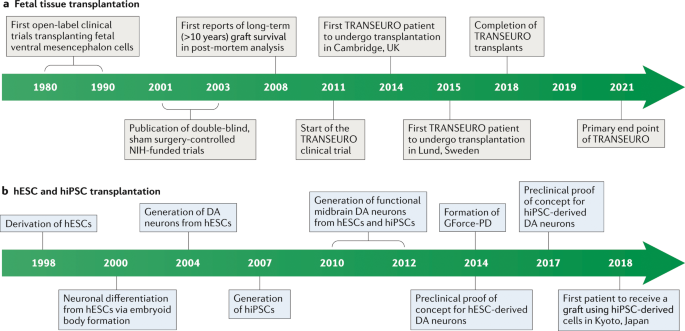




















Post a Comment for "Parkinson Disease Stem Cell Clinical Trials"